Kelhale is an extrafine
beclometasone formulation
With Kelhale, an extrafine beclometasone dipropionate particle formulation, patients can maintain a lower steroid dose than non-extrafine formulations.3
Kelhale can also help enable savings to the NHS.6,7
Kelhale is the least expensive extrafine beclometasone inhaler option for adult patients with asthma.7
Kelhale is competitively-priced across its range against non-extrafine beclometasone inhaler options for adult patients with asthma.7
Differentiating extrafine and non-extrafine ICS formulations and their benefits8
The Mass Median Aerodynamic Diameter (MMAD) of extrafine beclometasone dipropionate particles is substantially less than that of standard beclometasone dipropionate formulations with respective MMADs of 1.1 and 2.9 µm. Inhalation of such extrafine particles of beclometasone dipropionate results in greater deposition in small airways compared to large particles of beclometasone dipropionate.
A real-world study with over 30,000 patients has shown that beclometasone dipropionate in extrafine particle size produces real-life asthma treatment benefits.
Compared to large particle beclometasone dipropionate, use of the extrafine beclometasone dipropionate resulted in significantly higher:
- Asthma control
- Asthma control plus low short-acting-β2agonist (SABA) use
- Adherence to inhaled corticosteroid therapy
And significantly lower:8
- Rate of respiratory related hospitalisations
- Mean daily SABA dose
Extrafine particle beclometasone dipropionate inhaler use has demonstrated:8
- Improved real-life outcomes measures when managing asthma in primary care
- Greater small airway distribution and inhalation technique tolerance than large particle beclometasone dipropionate
- Equivalent asthma control and comparable tolerability at approximately half daily dose of large particle beclometasone dipropionate
Extrafine beclometasone products:
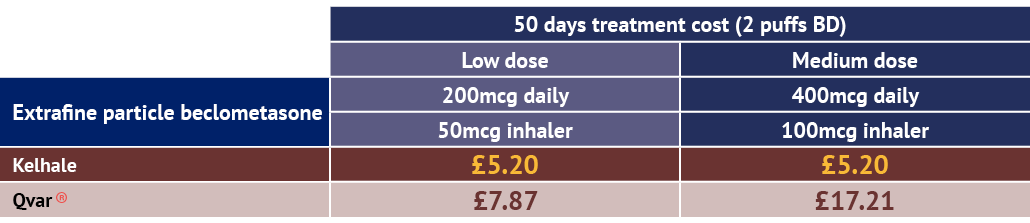
Adapted from the eMC Dictionary of Medicines and Devices (dm+d).7
Includes available branded pMDI competitors as listed in BNF November 2025.6
Non-extrafine beclometasone products:
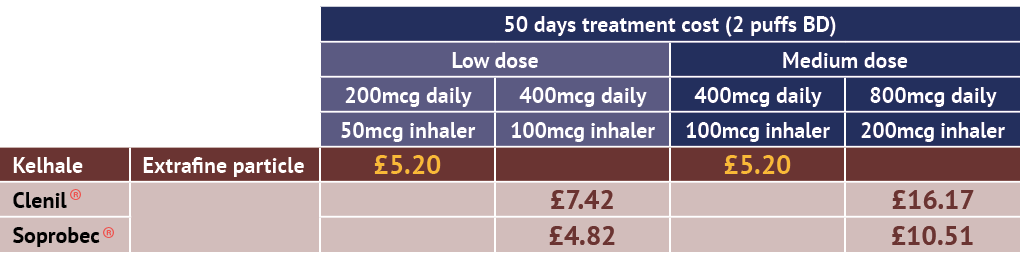
Adapted from the eMC Dictionary of Medicines and Devices (dm+d).7
Includes available branded pMDI competitors as listed in BNF November 2025.6
Qvar® is a trademark of Teva Pharmaceuticals. Clenil® is a trademark of Chiesi Ltd. Soprobec® is a trademark of Glenmark Pharmaceuticals.
Adverse event summary1,2
Adverse events which have been associated with beclometasone dipropionate include: Common (≥1/100 to <1/10); candidiasis of the mouth and throat, hoarseness, pharyngitis and taste disturbances. Uncommon (≥1/1,000 to <1/100); headache, vertigo, tremor, blurred vision, cough, increased asthma symptoms, nausea, urticaria, rash, pruritus, erythema and purpura. Serious hypersensitivity, paradoxical bronchospasm, and systemic effects of inhaled corticosteroids can also occur.
For full information about adverse events, contraindications, warnings and precautions please consult the Summary of Product Characteristics.1,2
Cipla's commitment to sustainable healthcare10
Established in 1935, Cipla is a global pharmaceutical company focused on sustainable growth with a firm commitment to make medicines accessible and available to those in need.10
Cipla has committed to meeting the following environmental targets in its production sites in India, where its respiratory range is manufactured.* By 2025 we aim to achieve:10
- 80% absolute reduction in Scope 1 (energy based) and Scope 2 emissions
- water neutrality
- zero waste to landfill
*India Manufacturing Operations includes manufacturing sites of Cipla Limited and our subsidiaries in India


